

Potassium is the most abundant of all elements in the human body and is essential for life. Scientists believe this breakthrough will lead to many more discoveries about how atoms work together and what they are capable of achieving when they interact with one another. This discovery can be attributed to recent improvements in technology, as it would have been difficult to measure these characteristics just a century ago. These findings will help scientists better understand how atoms interact with one another, which could have implications for future research in chemistry and physics. The study was conducted by scientists at the University of Stuttgart, who also found that its protons are closer to each other than those in any other atom. ^ "Empirical Definition & Meaning - Merriam-Webster".The element with the largest atomic radius is PotassiumĪ new study has concluded that potassium has the largest atomic radius out of all elements.^ Neon has van der Waal's radii thus its radii is the highest in its period.^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as at au av aw ax ay az ba bb bc bd be bf bg bh bi bj bk bl bm bn bo bp bq br bs bt bu bv bw bx by bz ca cb S."Atomic Screening Constants from SCF Functions.

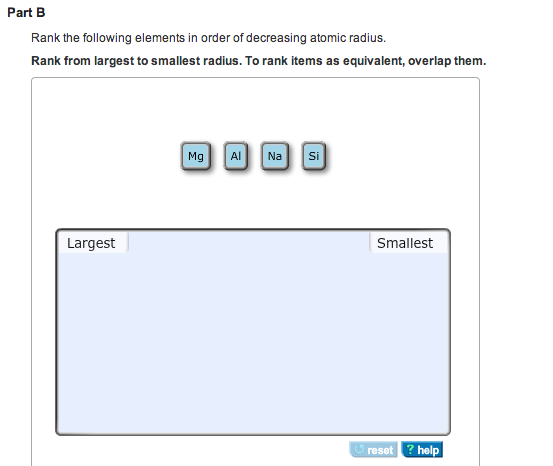
It basically means that you measured it through physical observation, and a lot of experiments generating the same results. Difference between empirical and experimental data: Empirical data basically means, "originating in or based on observation or experience" or "relying on experience or observation alone often without due regard for system and theory data".Covalent radius (Single-, double- and triple-bond radii, up to the superheavy elements.).These trends of the atomic radii (and of various other chemical and physical properties of the elements) can be explained by the electron shell theory of the atom they provided important evidence for the development and confirmation of quantum theory. The radius increases sharply between the noble gas at the end of each period and the alkali metal at the beginning of the next period. For instance, the radii generally decrease rightward along each period (row) of the table, from the alkali metals to the noble gases and increase down each group (column). Ītomic radii vary in a predictable and explicable manner across the periodic table. Under some definitions, the value of the radius may depend on the atom's state and context. Depending on the definition, the term may apply only to isolated atoms, or also to atoms in condensed matter, covalently bound in molecules, or in ionized and excited states and its value may be obtained through experimental measurements, or computed from theoretical models. Since the boundary is not a well-defined physical entity, there are various non-equivalent definitions of atomic radius. The atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron.


 0 kommentar(er)
0 kommentar(er)
